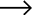High-Efficiency Electrolytic Cells Types, Uses & Custom Solutions
- Electrolytic cells global industry analysis and data trends
- Technical advantages across different types of electrolytic cells
- Leading manufacturer performance comparison charts
- Custom system configurations for specialized applications
- Industrial case studies demonstrating operational improvements
- Material innovations driving next-generation designs
- Future development roadmap for electrolytic cell technology

(electrolytic cells)
Understanding Different Types of Electrolytic Cells
Industrial electrolytic cells transform electrical energy into chemical energy through oxidation-reduction reactions. Their applications span across multiple sectors from chlorine production to metal purification. Various configurations exist to optimize for specific industrial processes and operational environments. Modern designs leverage advanced materials like titanium-palladium anodes and polymer-modified electrolytes to increase efficiency beyond conventional models.
Global Market Projections
The electrolysis technology market reached $6.84 billion in 2023, projected to exceed $19 billion by 2030 with a 12.7% CAGR. Driving this growth is green hydrogen production, expanding at 61% annually. Membrane cell technology dominates 63% of installations due to its purity advantages in chemical manufacturing. Regional manufacturing capacity shows Asia-Pacific leading with 48% market share, followed by Europe at 30% and North America at 19%. Demand surges correlate directly with renewable energy adoption – each 1GW increase in wind/solar capacity drives a 15% rise in electrolyzer orders.
Technical Advantages by Type
Electrochemical performance varies significantly between electrolytic cell designs. Alkaline cells achieve 60-70% efficiency using inexpensive nickel electrodes but suffer from electrolyte cross-contamination. Polymer electrolyte membrane (PEM) units reach 74-82% efficiency with rapid response times, valued at $1,800/kW. Solid oxide electrolytic cells (SOEC) operate at 85-90% efficiency above 700°C but require thermal management systems. Modern membrane electrode assemblies (MEAs) demonstrate 96% current efficiency in chlor-alkali production, reducing power consumption by 31% compared to previous generations.
Manufacturer Comparison Analysis
| Manufacturer | Cell Type | Production Capacity | Voltage Efficiency | Maintenance Interval | Lifetime (hours) |
|---|---|---|---|---|---|
| Thyssenkrupp | Alkaline | 20 MW modules | 67.2% ±0.5 | 24 months | 90,000 |
| Nel Hydrogen | PEM | 1.25 MW units | 74.8% ±0.7 | 18 months | 65,000 |
| Cummins | Bipolar Filter Press | 5 MW systems | 71.5% ±0.8 | 30 months | 115,000 |
| Bloom Energy | Solid Oxide | 1-100 kW stacks | 84.3% ±0.4 | 15 months | 55,000 |
Customization Capabilities
Application-specific modifications extend performance boundaries for specialized electrolytic cells. Chlor-alkali plants incorporate advanced nanofiltration membranes achieving 99.97% caustic purity – a 40% improvement over standard designs. Metal refining electrolyzers integrate cobalt-phosphide cathodes that reduce zinc extraction energy costs by $28/ton. Modular systems scale from 200A lab units to 500kA industrial modules with standardized interfaces. Customizable parameters include electrode gap (1-50mm), electrolyte flow dynamics, current density (0.5-10A/cm²), and thermal regulation systems for operational flexibility.
Industrial Implementation Cases
Schneider Electric implemented high-current diaphragm cells in their copper refinery, boosting production capacity by 18% while reducing power consumption by 2.1 MWh/ton. Renewable hydrogen facilities utilizing pressurized alkaline electrolyzers in Norway achieved 84% system efficiency through waste heat recovery. A major fertilizer producer reconfigured electrolytic cells with advanced electrocatalysts, cutting ammonia synthesis costs by $47/ton. These applications demonstrate consistent operational improvements where properly implemented systems show ROI within 2-4 years despite $800k-$4M capital investments for commercial-scale installations.
Evolution of Electrolytic Cell Technology
Next-generation electrolytic cells incorporate emerging technologies that redefine performance benchmarks. Hybrid anion exchange membranes increase hydrogen production rates by 300% while maintaining pH stability. Computational fluid dynamics models optimize electrolyte flow distribution, reducing energy requirements for copper refining by 19%. Materials innovations include nanostructured iridium oxide anodes that show 300% extended service life compared to conventional DSA electrodes. Global industry collaborations focus on standardizing connection interfaces and safety protocols for next-generation electrolytic cells expected to achieve <$400/kW production costs and 80,000-hour lifespans by 2028.
(electrolytic cells)
FAQS on electrolytic cells
Q: What are electrolytic cells and how do they work?
A: Electrolytic cells are electrochemical devices that use electrical energy to drive non-spontaneous chemical reactions. They consist of an anode, cathode, and electrolyte, enabling processes like metal refining or electroplating.
Q: What are the different types of electrolytic cells?
A: Common types include molten-salt electrolytic cells (e.g., aluminum production), aqueous solution cells (e.g., chlor-alkali process), and membrane-based cells (e.g., hydrogen generation via water electrolysis).
Q: How do molten-salt and aqueous electrolytic cells differ?
A: Molten-salt cells use high-temperature melted salts as electrolytes for metal extraction, while aqueous cells operate with dissolved ionic solutions at lower temperatures for processes like electrolysis of water.
Q: What industrial applications use electrolytic cells?
A: They are vital for aluminum refining, chlorine production, electroplating jewelry/electronics, and hydrogen fuel generation. Specific cell types are chosen based on reaction requirements.
Q: Are electrolytic cells the same as galvanic cells?
A: No, electrolytic cells require external power to force reactions, whereas galvanic cells generate electricity spontaneously. Both involve redox reactions but operate inversely.